The Early Cancer Institute is carrying out a number of research projects to evaluate new diagnostics and interventions.
Principal Investigators: Prof George Vasilliou, University of Cambridge and Dr Ted Brown, Oregon Health and Science University.
Funded by: ACED Project Award 2022
The myeloid malignancies are a group of related haematological cancers, including the myeloproliferative neoplasms, myelodysplastic syndromes and acute myeloid leukaemia.
Collectively, they affect 8-10 per 100,000 individuals per year and remain lethal to the majority, due to the lack of broadly effective treatments. Advances in our understanding of the pre-clinical evolution of myeloid malignancies have revealed that individuals at high risk of these cancers can be identified several years in advance through the mutational characteristics and clonal complexity of their shared ancestor, clonal haematopoiesis (CH).
This provided proof-of-principle that prevention of these lethal cancers may be a viable alternative to treatment. Notably mutations in splice factor genes (SF3B1, SRSF2 and U2AF1) and the gene for the epigenetic regulator ASXL1 are associated with a high risk of malignant progression. As such, individuals with mutations in these genes should be prioritised for future studies of early intervention/prevention treatments.
This project aims to:
i) identify and validate genetic/therapeutic vulnerabilities for high-risk CH driven by these genes using genetic and chemical screens
ii) decipher the impact of ASXL1 gene mutations in blood stem/progenitor cells and develop potent bioavailable inhibitors of histone ubiquitination as potential new treatments against ASXL1-mutant CH.
Principal Investigators: Mr Richard Mair and Rohit Sinha, University of Cambridge
Funded by: CRUK Early Detection Primer Award 2021
Educam is a novel study aiming to provide a completely non-invasive approach to the early detection of cancer, with a particular focus on brain cancer. Funded by the CRUK Primer Grant Award, this study will run in the Alliance for Cancer Early Detection (ACED) clinic in Cambridge from 2021, where all participants are eligible for recruitment. If successful, these methods could be used in GP surgeries longer term to provide broad coverage across the NHS.
Principal Investigator: Prof George Vassiliou, Professor of Haematological Medicine Wellcome-MRC Cambridge Stem Cell Institute
Funded by: Cancer Research UK Early Detection Project Award 2021
We are delighted to have been awarded a CRUK Early Detection Project Grant to study the phenomenon of clonal haematopoiesis (CH), the clonal expansion of blood stem cells and their progeny as a result of genetic mutations that give them a growth advantage over their normal, unmutated counterparts. CH is very common, but in a minority of cases progresses towards and eventually gives rise to acute myeloid leukaemia and related blood cancers. The project will follow and study a group of volunteers with CH over time in order to study how CH cells behave and what makes them expand and progress towards leukaemia. The overarching aim is to improve our ability to identify the characteristics of high-risk CH that is progressing towards leukaemia and develop ways to stop it from doing so. The project will be done in close collaboration with the ACED Clinic Cambridge.
Principal Investigators: Dr Robert Rintoul, Royal Papworth Hospital & Dr Nitzan Rosenfeld, CRUK Cambridge Institute
Funded by: Cancer Research UK Early Detection Programme Award
Diagnosing non-small cell lung cancer (NSCLC) at an earlier stage can save lives. Circulating tumour DNA (ctDNA) can be detected in plasma of patients with early-stage cancer, but it is unclear how early it can be detected, or what analytical sensitivity is required to detect the majority of stage I-II cancers in blood. Ideally, performance should be assessed in samples collected prior to diagnosis; however prospective collection in the general population is costly and complex. Previous studies collected plasma from patients at or after diagnosis, analysed few mutated loci, and detected ctDNA in only a minority of stage I cancer patients. Recent findings suggest an important role for methylation of plasma cell-free DNA in non-invasive detection of cancer. We hypothesize that multiplexed analysis in plasma samples of 100s-1000s of somatic mutations, combined with analyses of methylation and other molecular biomarkers, could detect early-stage cancer months or years ahead of clinical diagnosis in a high-risk population. To test this hypothesis, we bring together an array of cutting-edge technologies for cancer molecular diagnostics, study samples we collected at diagnosis of early-stage NSCLC, and establish a clinical study to collect samples from patients who have been treated with curative intent for NSCLC and are at high risk for either recurrent disease or a second primary cancer.
Principal Investigators: Dr Marc Tischkowitz, University of Cambridge; Dr Allison Kurian, Stanford University and Prof Gareth Evans, University of Manchester
Funded by: ACED Project Award 2020
Women with germline pathogenic variants in breast cancer genes have an increased risk of developing breast cancer. Some women may benefit more from early detection strategies than others, but risk estimates are currently not tailored to the individual. In this proof-of-principle study, Prof Evans and Drs Tischkowitz and Kurian will use a risk prediction model to calculate personalised risk of breast and ovarian cancer in women, based on their polygenic risk score, family history, as well as lifestyle and hormonal risk factors. The team will assess the feasibility and acceptability of incorporating personalised risk estimates into standard clinical practice, and they will investigate the influence of conventional and personalised risk estimates on women’s uptake of early detection and risk reduction.
Principal Investigator: Professor Fiona Gilbert, School of Clinical Medicine and Addenbrooke’s Hospital, Cambridge
Joint Lead Applicant: Professor Paul Pharoah, Department of Public Health and Primary Care
Funded by: Cancer Research UK Early Detection Programme Award
A team led by Fiona Gilbert, Professor of Radiology, and Paul Pharoah, Professor of Cancer Epidemiology, will look at risk-adaptive breast cancer screening, exploring whether it is possible to stratify women into different categories of breast cancer risk and offer them a more appropriate screening test based on this. Women at increased risk of breast cancer, which will be assessed by age, breast density, inherited predisposition to the disease and lifestyle / hormonal risk factors, may benefit from more detailed imaging tests such as abbreviated MRI, contrast enhanced spectral mammography and automated whole breast ultrasound. The results from the study will enable the NHS breast screening programme to offer women at greater risk more appropriate imaging tests to detect their cancer at an earlier stage, ultimately leading to improved survival. Read more
Principal Investigator: Professor Doug Easton, Centre for Cancer Genetic Epidemiology,
Joint Lead Applicant: Dr Nitzan Rosenfeld, CRUK Cambridge Institute
Funded by: Cancer Research UK Early Detection Programme Award
The team, led by Doug Easton, Professor of Genetic Epidemiology, and Nitzan Rosenfeld, Group Leader, CRUK Cambridge Institute, will establish a cohort of women at high risk of breast and ovarian cancer to investigate the potential to use DNA in blood or cervical samples for the early detection of the two cancers using state-of-the-art methodology. The research will establish whether current methods of ctDNA analysis, using either blood or cervical samples, are sufficiently sensitive to have utility in early detection and will determine the relative sensitivity in different risk groups. It is hoped that the results will inform the design of future trials to establish the utility of such methods in clinical practice.
Principal Investigator: Dr Serena Nik-Zainal, MRC Cancer Unit
Funded by: Cancer Research UK Early Detection Project Award
Mutational-signatures in human cancers are the final outcome of combinations of DNA damage and DNA repair processes. Direct DNA damage incurred by tobacco smoke, UV light or other environmental mutagens and direct abnormalities of a DNA repair pathway like homologous recombination (HR) repair or mismatch repair (MMR) leave characteristic imprints on the genome. However, abnormal cellular processes like replication stress can result in mutagenesis as well, albeit indirectly. Mutational-signatures are therefore simply a read-out of a spectrum of cellular abnormalities and can thus be thought of and exploited in that way. In this Early Detection Project, we aim to identify on-going mutational-signatures that can be used as a surrogate for particular cancer development processes and determine signatures that may be informative for therapeutic intervention. Apart from identifying these clinically-relevant mutational signatures, we aim to design mutational-signature-based clinical assays that could be used for early detection. We will test our assays, optimize their performance and demonstrate how well they perform in a clinical screening setting.
Chief Investigator: Professor Rebecca Fitzgerald
Principal Investigators:
Dr Massimiliano di Pietro, gastric and oesophageal cancers
Miss Victoria Snowdon, liver cancer
Sponsor's coordinating Investigator: Dr Marc van der Schee
Funded by: Owlstone Medical Ltd (sponsors) and CRUK Cambridge Centre
The PAN-cancer study is a collaboration between the Cancer Research UK Cambridge Centre, Owlstone Medical Ltd and Cambridge University Hospitals NHS Foundation Trust. The study is evaluating whether Owlstone Medical’s Breath Biopsy® technology can differentiate between patients with and without different cancer types by comparing breath biomarkers in gastric, oesophageal and liver cancer and healthy volunteers. This study is the first step in evaluating volatile organic compounds analysis as a test to improve early detection rates for cancer with future applicability to primary care.
The PAN-cancer study is a collaboration between the Cancer Research UK Cambridge Centre, Owlstone Medical Ltd (sponsors) and Cambridge University Hospitals NHS Foundation Trust to evaluate Owlstone Medical’s Breath Biopsy® technology.
Further information can be found on the Owlstone Medical PAN Cancer Early Detection website.
Principal Investigator: Professor Fiona Gilbert, Department of Radiology
Co- Investigator: Dr Sarah Bohndiek, Department of Physics
Funded by: Cancer Research UK Cancer Imaging Centre in Cambridge and Manchester
For more information about this project, please visit this website.
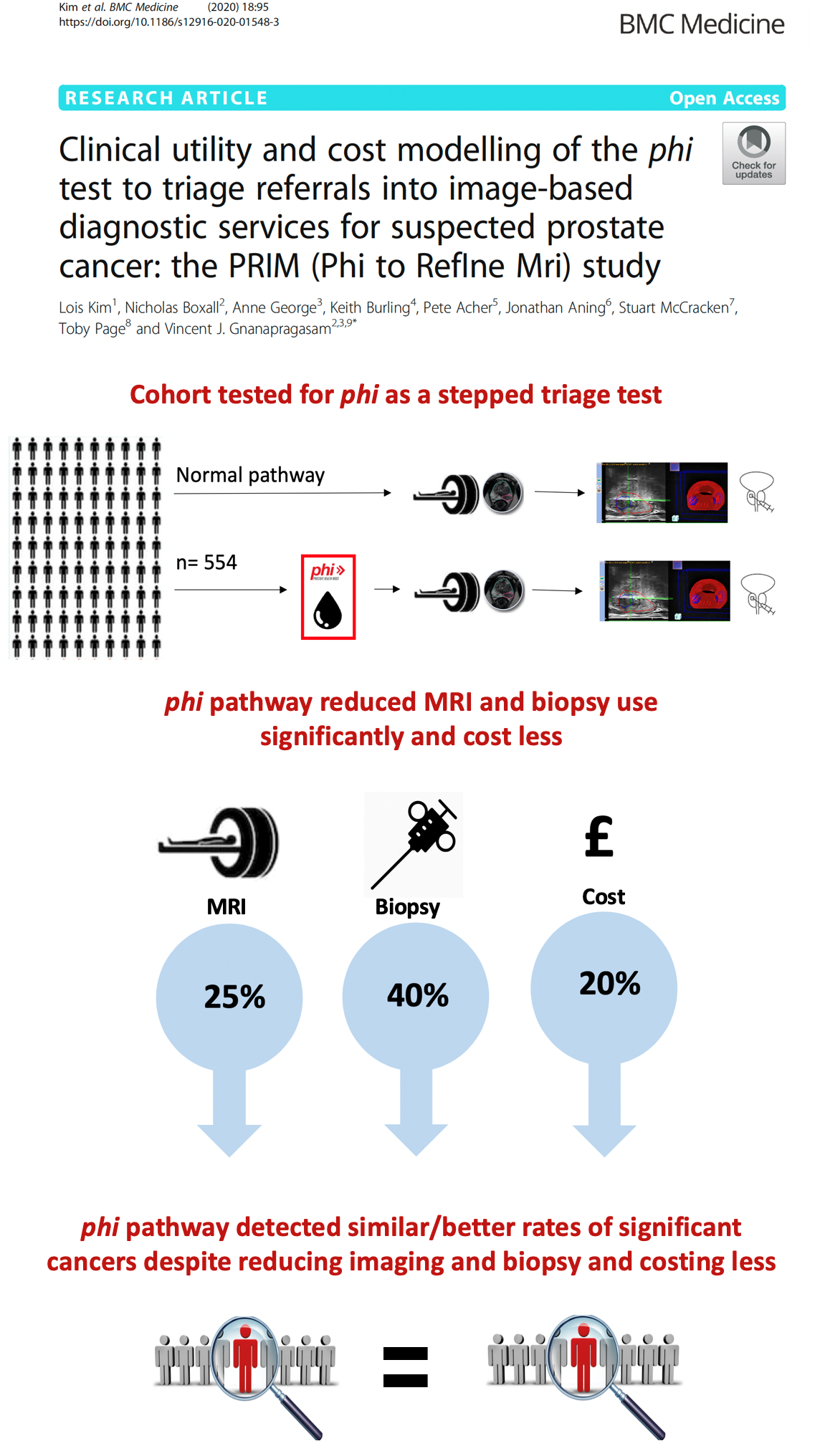
Principal Investigator: Professor Vincent Gnanapragasam, Department of Surgery
Funded by: Addenbrooke's Charitable Trust & CRUK Cambridge Centre Urological Malignancies Programme
Published Paper: Clinical utility and cost modelling of the phi test to triage referrals into image-based diagnostic services for suspected prostate cancer: the PRIM (Phi to RefIne Mri) study.
BMC Medicine, published April 17 2020. DOI: 10.1186/s12916-020-01548-3
The Prostate Health Index, which combines total PSA (tPSA), free PSA and [-2]proPSA, has been shown to outperform tPSA for the prediction of prostate cancer on biopsy. The PRIM study (PHI in Refining MRI) is recruiting in five UK Centres, including the Department of Urology at Cambridge University Hospitals Trust. The study, led by Professor Vincent Gnanapragasam, determines how the test can be used to reduce and refine the need for expensive imaging tests, such as MRI, while increasing the detection of lethal prostate cancers. The target population was those men with a first referral for suspected new prostate cancer. PRIM represented one of the first multi-centre UK studies to combine a biomarker with modern MR imaging to refine early detection of prostate cancer and applied in real world clinical practice. The data published in 2020 showed that the use of the PHI as a triage test could reduce both imaging and biopsies needs by a quarter while maintaining diagnostic efficiency using two definitions of clinically significant prostate cancers. We further demonstrate that introducing the PHI is likely to be both the cheapest per referred patient and cheapest per net tumour detected allowing resources to be diverted to other needs.
Principal Investigator: Dr Sarah Bohndiek, Department of Physics
Co-Investigator: Professor Rebecca Fitzgerald, MRC Cancer Unit & Dale Waterhouse, Department of Engineering
Collaborator: Dr Massimiliano di Pietro, MRC Cancer Unit
Funded by: Cancer Research UK & Stand Up to Cancer
The results of the study can be found in the paper published in the Journal of Biomedical Optics, 26(10), 106002 (2021). https://doi.org/10.1117/1.JBO.26.10.106002: First-in-human pilot study of snapshot multispectral endoscopy for early detection of Barrett’s-related neoplasia
This multidisciplinary research project has developed the novel application of a multispectral imaging camera for use in endoscopy to detect early pre-cancerous changes in the oesophagus of Barrett’s oesophagus patients. This clinical feasibility study in 20 patients tests level of confidence in delineating the area of interest by the multispectral endoscope in terms of image quality and visibility.
For more information about this project, please click here. More information about the clinical trial can be found here.
Principal Investigator: Dr Massimiliano di Pietro, MRC Cancer Unit
Co-Investigator: Professor Lorenz Wernisch, MRC Biostatistics Unit
Funded by: Cancer Research UK Cambridge Centre Early Detection Programme Pump Priming Awards 2016
Oesophageal cancer has poor prognosis and limited response to standard oncological and surgical therapies. The commonest cancer type in the Western world is the adenocarcinoma, which is normally preceded by a precancerous condition known as Barrett’s oesophagus (BO).
For this reason patients with BO are followed up with regular endoscopies and multiple random biopsies to allow diagnosis of dysplasia, which can be treated endoscopically to prevent cancer progression.However, dysplasia and often early cancer can be invisible at standard endoscopy and missed even by multiple biopsies. In addition, there is a lack of reliable tests to predict which patients with BO are at higher risk of cancer.
Advanced endoscopic technologies and tissue molecular tests offer a potential solution to this problem. Our previous research suggests that auto-fluorescence imaging (AFI), in combination with molecular tissue biomarkers and in vivo microscopic detection of dysplasia by confocal endo-microscopy allows accurate diagnosis, with the potential to improve patient risk stratification.
With a randomized study, we are seeking confirmation of this novel diagnostic algorithm. This has the potential to revolutionize the diagnostic approach to BO by in-vivo endoscopic diagnosis and molecular stratification in a single clinical intervention, to allow early treatment before cancer progression.
Principal Investigator: Dr Marc Tischkowitz, Department of Medical Genetics
Co-Investigator: Professor Paul Pharoah, Department of Public Health and Primary Care
Funded by: Cancer Research UK Cambridge Centre Early Detection Programme Pump Priming Awards 2016
This project aim is to develop a national framework to identify and monitor individuals and families with high penetrance inherited cancer syndromes. Such individuals have (on average) a greatly increased risk of cancer and frequently develop multiple tumours. Hence trials of early detection (e.g. imaging, circulating tumour DNA etc.) can be more efficiently performed in a small number of high risk individuals than in larger numbers of individuals at population risk.
This addresses one of the major challenges in Early Detection, namely the ability to target early detection approaches to appropriate high risk populations.
Principal Investigator: Dr Robert Rintoul, Papworth Hospital
Co-Investigator: Dr Yoryos Lyratzopoulos, Department of Public Health and Primary Care
Funded by: Cancer Research UK Cambridge Centre Early Detection Programme Pump Priming Awards 2016
The results of the study can be found in the paper published in Thorax 2019;74:466-472. Incidence of second and higher order smoking-related primary cancers following lung cancer: a population-based cohort study: doi.org/10.1136/thoraxjnl-2018-212456
This project addresses an emerging problem in cancer prevention and control – the development of second primary cancer in survivors of previous upper aero-digestive cancer (lung, head and neck, oesophagus). This new challenge has arisen because of the welcome and substantive improvement in cancer survival, but it calls for new research and interventions to mitigate that risk.
Building on pilot work examining outcomes in head and neck cancer survivors who develop second primary cancers, this project will examine the risk patterns and variation in risk of developing smoking-related cancers in survivors of lung, oesophagus and head and neck cancer. This project also provides an opportunity for laboratory, clinical, epidemiological and health economics enquiries to characterize mechanisms and risk patterns and develop interventions to minimise the risk of second cancer.
It will also allow us to begin to develop methods for early detection of second primary cancers with a view to improving outcomes. This work will inform plans to establish a cohort of upper aero-digestive survivors who could be studied prospectively to develop biomarkers related to individual disease risk and early diagnosis and evaluate new preventative, diagnostic and therapeutic interventions and models of care across primary, secondary and tertiary healthcare.